Introduction
Resistance to apoptosis is a hallmark of cancer, and modulation of BCL-2 family proteins is an important mediator of such resistance in hematologic malignancies. Despite the clinical efficacy of the BCL-2 inhibitor venetoclax (VEN), prolonged treatment may lead to resistance, such as the BCL2 G101V mutation (Blombery et al, Blood, 2020); however, over half of VEN resistant cases are not explained by known genetic mechanisms. Phosphorylation of BCL-2 at serine-70 (S70pBCL2) or of MCL-1 at threonine-163 (T163pMCL1) have been shown to increase sequestration of the pro-apoptotic protein BAX (Deng et al, JNCI, 2000) and stabilize the level of anti-apoptotic protein MCL-1 (Wang et al, Mol Cancer, 2014), respectively. We hypothesized that the increase in post-translational modifications of BCL-2 family members, in particular S70pBCL2 and T163pMCL1, are novel mechanisms of functional VEN resistance in lymphoid malignancies. We further hypothesized that the FDA-approved phosphatase activator drug FTY720 (fingolimod) would de-phosphorylate these BCL-2 family members and thereby re-sensitize malignant lymphoid cells to VEN-induced apoptosis.
Methods
A VEN resistant diffuse large B-cell lymphoma cell line (OCI-Ly1-R) as well as peripheral blood mononuclear cells from 12 previously untreated CLL patients co-cultured with human stromal NK-Tert cells were treated ex vivo with VEN +/- FTY720. A VEN sensitive cell line (OCI-Ly1-S) was treated with VEN +/- a phosphatase inhibitor okadaic acid (OA). Western blot was used to evaluate changes in S70pBCL2 and T163pMCL1 protein levels. BH3 profiling via flow cytometry was performed to determine the survival dependence on anti-apoptotic BCL-2 family members via cytochrome c release in response to specific BH3-only peptides and drugs such as VEN applied directly to mitochondria (Ryan et al, Biol Chem, 2016). Cell viability assays (CellTiter-Glo, Trypan Blue and Annexin/Hoechst) were employed to investigate the effects of FTY720 on OCI-Ly1-R resistance to VEN. The BCL-2-BAX interaction was investigated using co-immunoprecipitation in VEN resistant and sensitive cells following treatment with VEN +/- FTY720. T-test, ANOVA and multiple comparison with a statistical significance set at 2-tailed p ≤ 0.05 were used.
Results
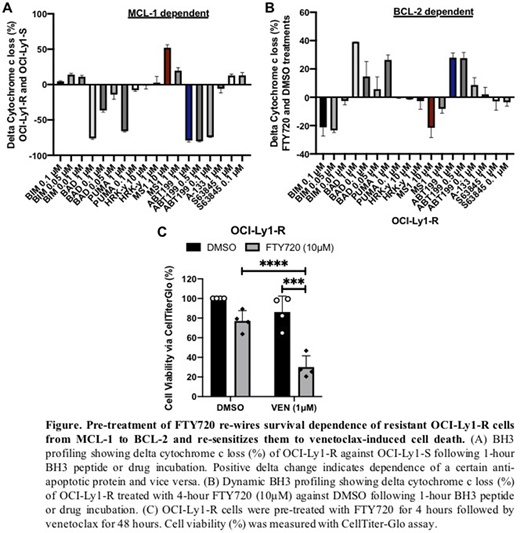
OCI-Ly1-R cells displayed higher S70pBCL2, T163pMCL1 and MCL-1 expression compared to OCI-Ly1-S cells. Notably, the increase in S70pBCL2 was associated with reduced response of VEN-mediated BCL-2-BAX dissociation, while the increase in T163pMCL1 was accompanied by enhanced MCL-1 protein expression. Using BH3 profiling, we found that the increase in S70pBCL2, T163pMCL1 and MCL-1 expression were functionally associated with a decrease in BCL-2 survival dependence (-79.1%, 1μM VEN, P < 0.0001) and an increase in MCL-1 dependence (+52%, 10μM MS1, P < 0.0001) (Fig. A). The addition of FTY720 reversed these observations in OCI-Ly1-R cells, where we observed a decrease in S70pBCL2, T163pMCL1 and MCL-1 protein expression, BCL-2 and BAX interaction, as well as a "re-wired" functional dependence toward BCL-2 (-21.6% 10μM MS1, +27.9% 1μM VEN, P < 0.0001) (Fig. B).
Importantly, pre-treatment with FTY720 re-sensitized OCI-Ly1-R cells to VEN-induced cell death (+56.1%, P = 0.0001) (Fig. C). Conversely, treatment with a phosphatase inhibitor, OA, led to an increase in S70pBCL2, T163pMCL1 and MCL-1 expression as well as reduced late death of OCI-Ly1-S cells (-60%, P = 0.0018). We validated our cell line results in primary CLL cells, and again the combination of FTY720 and VEN similarly reduced S70pBCL2, T163pMCL1 and MCL-1 expression, increased BCL-2 dependence, and enhanced VEN-induced cell death (+23.6%, P < 0.0001).
Conclusion
Increased S70pBCL2 and T163pMCL1 are associated with VEN resistance, in part by inhibiting VEN-induced BCL-2-BAX dissociation and switching the functional survival dependence from BCL-2 to MCL-1. FTY720 re-sensitizes VEN resistant cells by reducing S70pBCL2, T163pMCL1 and MCL-1 expression, dissociating BAX from BCL-2 and "re-wiring" the survival dependence to BCL-2. These preclinical findings support the exploration of this strategy clinically in patients with VEN resistant lymphoid malignancies.
Davids:Sunesis: Consultancy; AbbVie: Consultancy; Surface Oncology: Research Funding; Genentech: Consultancy, Research Funding; Eli Lilly: Consultancy; Celgene: Consultancy; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy; Ascentage Pharma: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy; Pharmacyclics: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Verastem: Consultancy, Research Funding; Zentalis: Consultancy; Novartis: Consultancy, Research Funding; Gilead Sciences: Consultancy; Bristol Myers Squibb: Research Funding; Janssen: Consultancy; MEI Pharma: Consultancy, Research Funding; Syros Pharmaceuticals: Consultancy; Merck: Consultancy; Research to Practice: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.